Brief summary
This article delves into the intricate effects of UV degradation, commonly known as chalking, on PVC materials. It investigates the progressive deterioration of PVC properties under UV exposure, outlining key phenomena such as discoloration, reduction in mechanical strength, and visible surface changes. By exploring the mechanisms behind chalking and its implications, we sheds light on the challenges posed by UV weathering in PVC applications.
What is chalking?
Chalking is a phenomenon that occurs when UV degradation starts at the surface of the PVC and gradually penetrates deeper. As the outer layer of PVC degrades, an increasing number of particles of pigments like TiO2 and other inorganic ingredients like CaCO3 are released, forming a white, powdery deposit. This deposit gives the pipe a whitish appearance. The white powder is then washed away by rain or water sprays, exposing the underlying layers to further degradation and reducing the material's strength.
While a small amount of chalking can be tolerated and may even be desirable in white PVC products, it has a negative effect on gloss. Chalking is completely unacceptable in dark-colored materials.
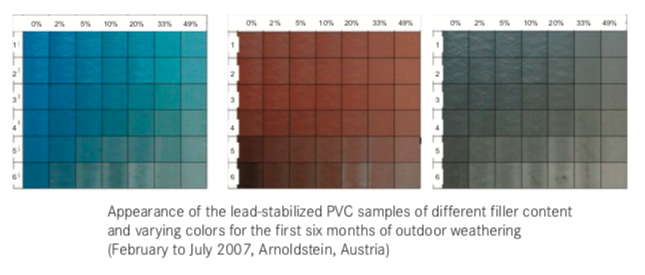
Are UPVC Pipe & profiles UV Resistant?
The UV resistance of rigid PVC pipes and profiles depends on the level of UV stabilization and the quality of the materials used. Compounds that are not UV-stabilized tend to degrade quickly, leading to the loss of mechanical properties and visible effects like bleaching, darkening, opacification, cracking, and shrinking.
In addition to damage caused by UV radiation between 290–400 nm, visible light between 400–500 nm also has a significant effect. Visible light at this wavelength has enough energy to damage weaker structures such as:
- Double bonds
- Conjugated double bonds
- Branched structures
During weathering, white compounds tend to turn yellow and then bleach, while colored compounds will fade.
The consequences of sunlight exposure for UPVC pipe
Sunlight exposure has several consequences on rigid PVC pipes:
-
Initial and long-term discoloration
Initial discoloration occurs after about 200 hours of exposure. With prolonged exposure, the mechanical erosion of the more photo-oxidized layers regenerates the surface. If the erosion is efficient enough, the profile may never enter the discoloration phase. The mechanical properties of lead-stabilized compounds are better than those of tin mercaptide-based compounds.
TiO2 in the PVC matrix has a refractive index of 1.83, while TiO2 exposed to air has a refractive index of 2.71. As the PVC surface oxidizes, a higher percentage of TiO2 becomes exposed to air, increasing opacity and brightness in the exposed areas.
-
Structure, flexibility, and strength
UV degradation leads to dehydrochlorination and a reduction in the molecular weight of PVC, accompanied by yellow-brown discoloration. This is followed by a steady decrease in tensile strength, modulus of elasticity, impact resistance, and flexibility, resulting in a loss of mechanical properties. The primary effect is due to photo-oxidation, which causes more chain scission than chain cross-linking.
-
Anomalous yellowing of TiO2 in chalking
All TiO2-stabilized PVC products tend to yellow with increasing TiO2 concentration. With more initial yellowness due to higher TiO2 content, it is not unexpected that due to chalking, there is a greater impact on the perceived whiteness.
TiO2 exhibits maximum gloss retention at about 10 phr. If gloss retention is less critical, then the grade of TiO2 becomes less relevant.
Prevention of UV discoloration
To prevent discoloration caused by UV radiation, UV stabilizers and materials such as gaskets can be used to reduce the direct exposure of PVC surfaces to sunlight. Additionally, selecting the right materials can significantly reduce degradation effects.
Gaskets used in rigid PVC systems can be designed to provide additional protection against UV light, preventing the degradation of the PVC surface and improving the system's long-term durability.
Credits: Yashodhan Kanade
_5f116712726a4.jpeg)



